In this blog post, Eric Bollinger provides a brief overview of their recently published paper regarding the effects of antibiotics on methane production in sediments. The study links topics from ecotoxicology, environmental physics, and molecular ecology in the context of climate change.
Methane in freshwaters
Methane (CH4) is a greenhouse gas with a 28-fold higher global warming potential than carbon dioxide (CO2). It is inter alia produced in anoxic layers of freshwater sediments, where Archaea (i.e., single-celled microorganisms) mainly utilize acetate or H2/CO2 catabolized from organic matter as a substrate for methanogenesis. Both anthropogenic and natural CH4 emissions are rising which can only be partially explained by climate change feedback or (agrarian) nutrient input into freshwater systems. To our surprise, no study has yet considered omnipresent chemical stressors (e.g., via antibiotics) as a potential influence for natural CH4 production.
Antibiotics
Antibiotics are used in veterinary and human medicine and are mainly introduced into freshwaters via wastewater treatment plants. Average concentrations are in the lower µg/L range with extreme sites surpassing even multiple mg/L. Being designed to target bacterial cell processes, negative effects on growth, functioning, and community composition are reported. Hence, the researchers considerthat it is not far to seek to select antibiotics as stressors in their pilot study assessing chemical stress effects on natural CH4 production.
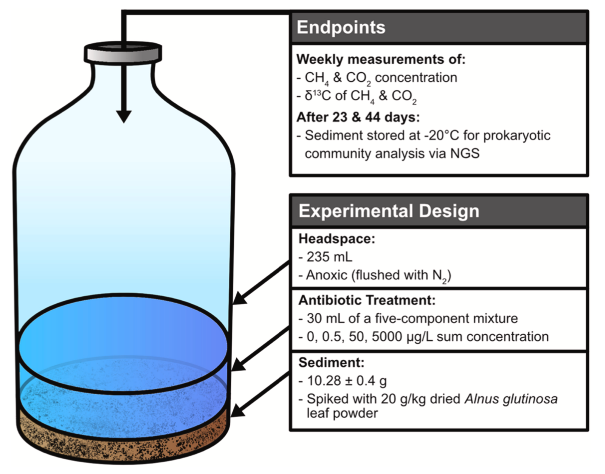
Study design
Natural pond sediment was spiked with pulverized black alder leaves and incubated in an anoxic system at four levels of an antibiotic mixture. Concentrations and 13C/12C isotopic ratios of CH4 and CO2 were measured weekly to inform about production ratesas well as the dominant pathway of production (i.e., from acetate or H2/CO2). Furthermore, eukaryotic and prokaryotic communities were assessed via metabarcoding of the 16S rRNA gene.
Study highlights
Replicates from all antibiotic treatments showed the same regime of CH4 production. First, CH4 production is limited by the availability of more favorable electron acceptors (~first three weeks). Once these electron acceptors are used up, CH4 production increases exponentially (~week three to five) until reaching a steady-state, which is determined by the amount of available substrates. Unexpectedly, production rates of CH4 were up to two-fold higher in presence of antibiotics. Since 13C/12C ratios indicate a comparable substrate utilization between all treatments, the most likely explanation is a community shift as indicated by metabarcoding results.
Associated project
The study is the basisfor the project “HyPro” funded by the Carl Zeiss foundation attracted by Jun.-Prof. Dr. MircoBundschuh (University Koblenz-Landau) and Jun.-Prof. Dr. Sabine Filker (TU Kaiserslautern), which extends on the addressed subject with potential options for theses. For more information contact bollinger@uni-landau.de or bundschuh@uni-landau.de.
The paper was authored byEric Bollinger, Jochen Zubrod, Foon Yin Lai, L. Ahrens, Sabine Filker, Andreas Lorkeand Mirco Bundschuh. This study has been published under open-access license in Ectoxicology and Environmental Safety Volume 228 .
